Pharmaceutical Marketing Case Study
End-to-End Clinical Trial Recruitment for a Global Pharma Brand
We help pharmaceutical companies educate and engage healthcare professionals and patients. By creating compliant, informative campaigns and content, we improve visibility and brand awareness. Our strategies drive measurable results while maintaining credibility in a regulated space.

Comprehensive Pharmaceutical Workflow From Strategy to Enrollment
This clinical trial campaign followed a structured pharmaceutical workflow designed for compliant patient recruitment. From protocol review and audience definition to creative production, MLR/IRB approvals, and ongoing optimization, we delivered a multi-channel strategy that respected regulatory requirements while meeting performance goals.
To support performance, we implemented a multi-channel recruitment strategy combining high-intent search traffic, broad-reach social advertising, contextual placements on Reddit, and geofencing near clinical trial sites. Our channel mix ensured that patients and caregivers discovered compliant study information at meaningful points in their decision-making journey. Each tactic was continuously optimized through A/B testing, segmentation adjustments, and data-driven refinement.
Throughout the campaign, we prioritized privacy, compliance, and transparent communication. No personal health data was collected or used for targeting, and all messaging maintained neutral, fact-based language consistent with pharmaceutical guidelines. By integrating scientific accuracy with marketing best practices, we delivered a recruitment ecosystem that increased trial visibility, supported site engagement, and achieved measurable performance goals while respecting industry regulations.
Campaign Foundations
Strengths
- Entire campaign uses only pre-approved, compliant language.
- No personal data collection or health profiling, protecting privacy.
- Channel mix balances high-intent search with broad reach (social) and contextual awareness (Reddit & geofencing).
- Landing page is optimized for clarity, conversion, and compliance.
Optimizations
- A/B testing ad variations across platforms to identify top-performing creative and messaging.
- Refining audience segments by platform (e.g., caregivers on Meta, patients on Reddit).
- Phased budget deployment: Testing → Optimization → Scaling.
- Adjusting by region and site enrollment progress as needed.
- Monitoring CTR and engagement to guide spend reallocation.
Opportunities
- Maintain use of pre-approved, compliant language across all assets.
- Continue privacy-safe tactics with no health profiling or PII capture.
- Leverage our experience managing regulatory-safe recruitment ads to scale into new indications and geographies.
- Expand the proven channel mix of search, social, Reddit, and geofencing into additional markets.
- Further optimize the landing experience for clarity, conversion, and compliance.
Awareness
- Study promoted across coordinated search, display, and social channels.
- Language clearly defines epNEC and the DLL3 biomarker to improve understanding.
- Trial sites and eligibility are consistently highlighted.
- Reddit, Meta, and Google all carry cohesive, approved messaging.
- ClinicalTrials.gov listing (NCT05882058) is reinforced as the primary information source.
Pharmaceutical & Health Design with Compliance Expertise
Pharma Compliance & Regulatory
Our pharma and healthcare work is built on a rigorous regulatory foundation. We translate clinical evidence and regulatory requirements into compliant, patient-focused communications that can move smoothly through MLR, IRB, and global approval workflows.
This process adapts easily to the platforms your teams already use, ensuring controlled content creation, review, versioning, and global approvals at every stage.

Patient-Facing Educational Materials
- Partnered with clinical teams to prepare patient-facing educational materials and recruitment tools reflecting proven safety and efficacy from clinical trials.
- Secured Institutional Review Board (IRB) approvals to ensure all materials met ethical standards.
- Supported Medical, Legal, and Regulatory (MLR) review for promotional and educational assets required for FDA approval and U.S. launch.
- Ensured Important Safety Information (ISI) and risk disclosures were accurate and prominent.
- Prepared assets for international market readiness.
Market Access Materials
Objective:-
- Led design and content development of patient-facing materials explaining treatment options and clinical trial participation.
- Ensured each asset was compliance- and IRB-approved, with ISI integrated for clarity and patient safety.
- Collaborated with medical, legal, and regulatory (MLR) teams to secure approvals efficiently and document every step for audit readiness.
Outcome:-
- Applied plain-language principles and intuitive layouts to improve patient engagement and understanding.
- Built in privacy protections and ensured alignment with HIPAA/GDPR standards where applicable.
- Coordinated reviews, managing comments, version control, and final sign-offs.

Digital Compliance
Landing page optimized for compliance, clarity, and conversion. The landing page:
- Explains the trial purpose and eligibility in plain language.
- Features an eligibility checklist, site map, and clear call to action.
- Includes a direct link to the ClinicalTrials.gov entry.
- Avoids personal data collection or unverified health claims.
- Uses neutral, regulatory-compliant language.
Interested individuals are routed to contact local investigative sites directly—helping protect privacy, streamline approvals, and avoid HIPAA complications by avoiding storage of unverified claims.
Campaign Overview
Ad Spend Total
$150,000
An overview of each campaign’s reach, engagement, and cost.
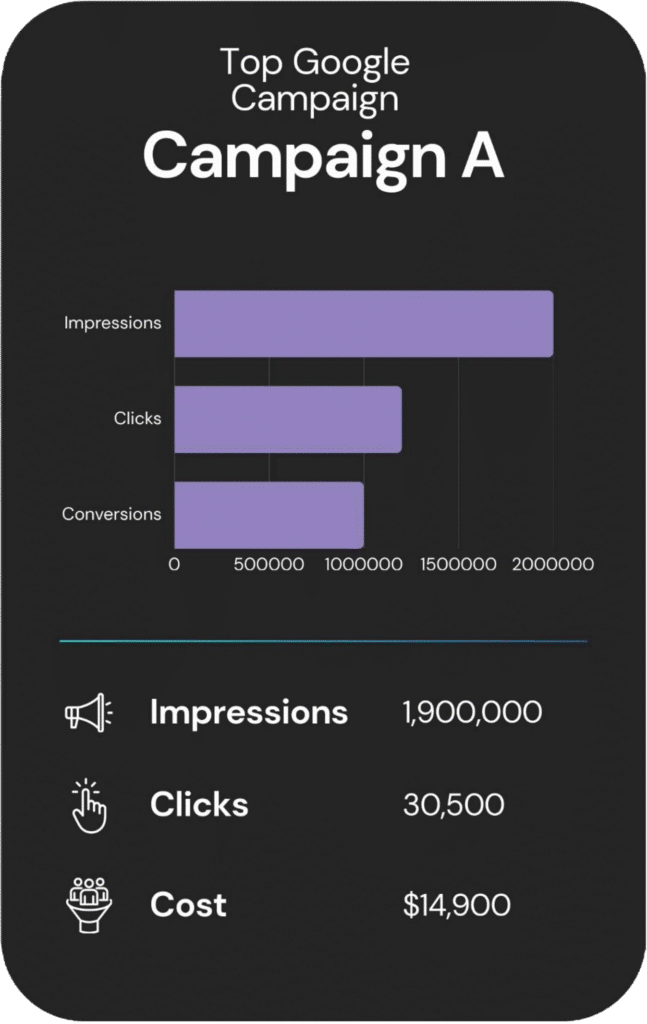
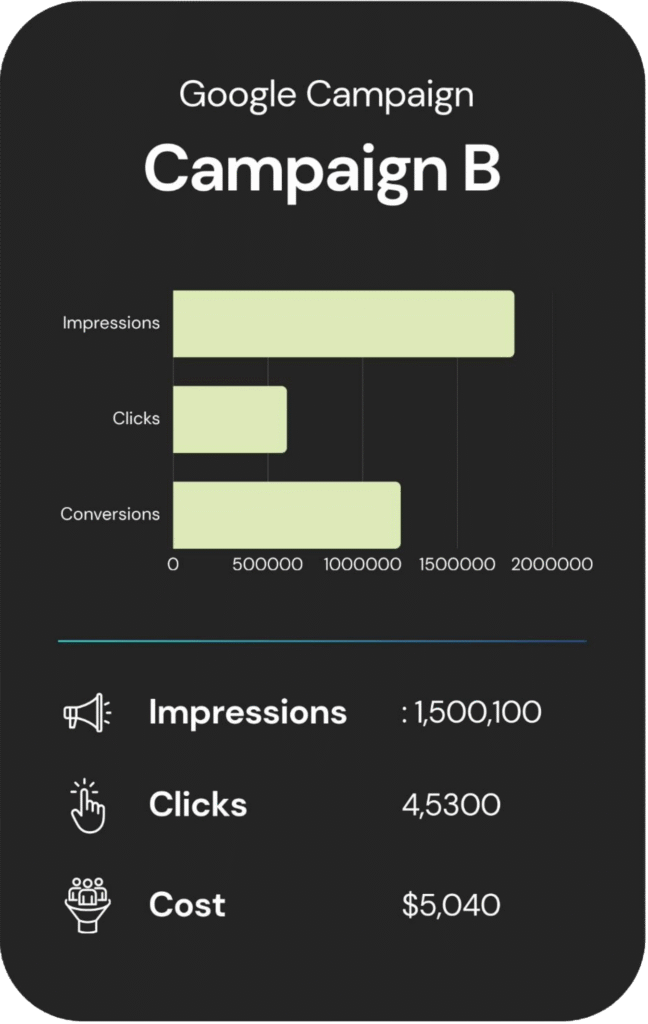
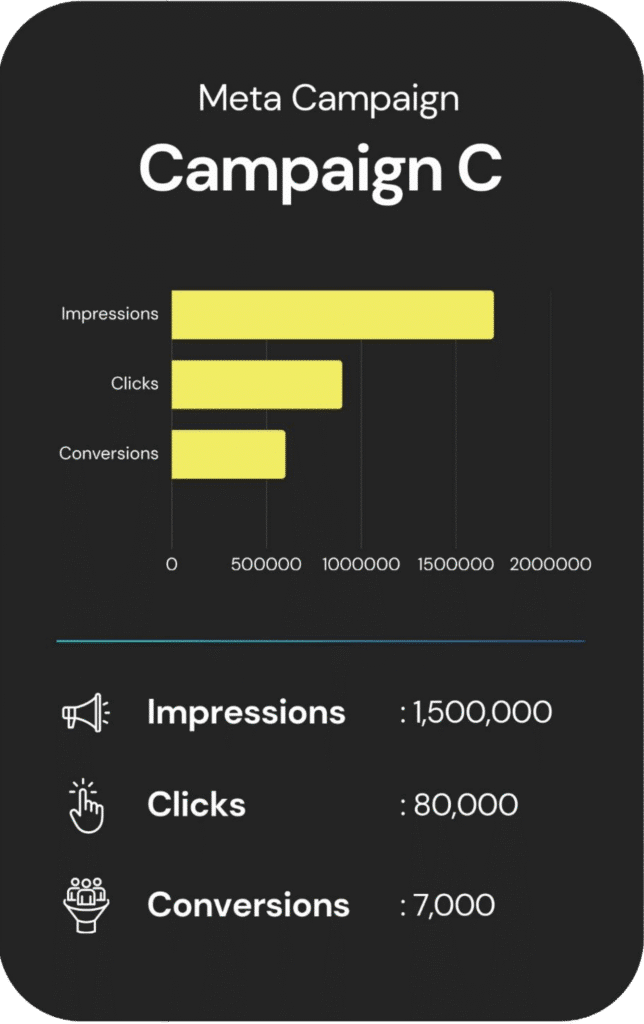
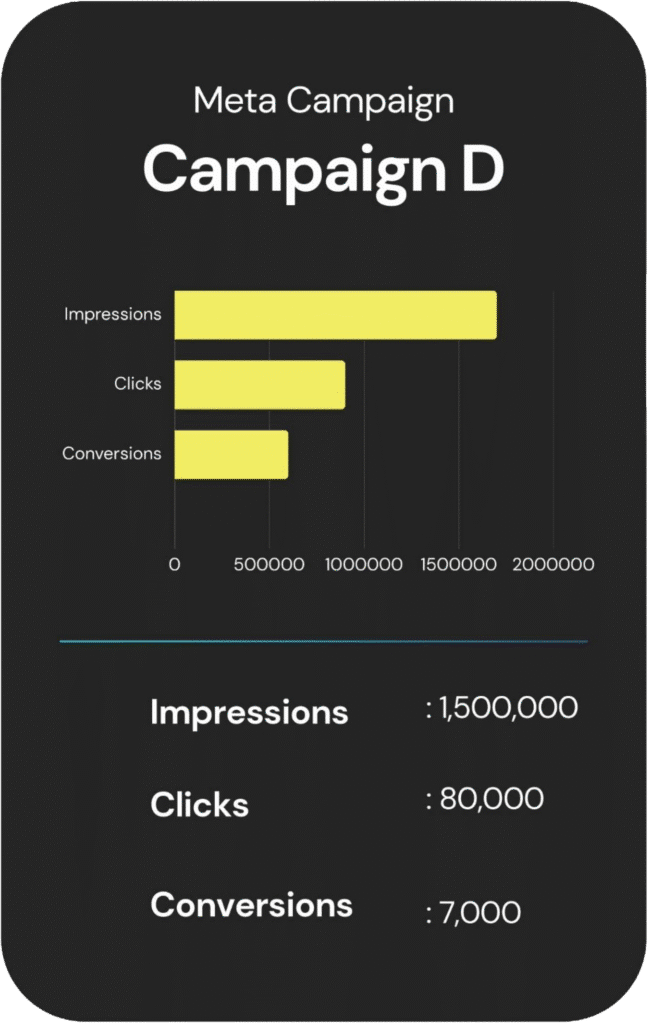
Other Services
1. Google Ads & Paid Campaign Management
2. Search Engine Optimization (SEO)
3. Website Design, Hosting & Domain Management
4. Social Media Management
5. Online Reputation Management
6. Content Strategy & Digital Branding
7. Email Marketing & Automation
8. Graphic Design & Visual Branding
Cookie Management & Tracking
Outcome:-
- Implemented compliance-approved templates, maintaining digital tracking for campaign performance analysis.
- Supported the internal compliance team on website tracking and consent templates for a clinical research study.
- Worked with the team to obtain approvals for all site content and tracking cookies (e.g., _gcl_au, test_cookie, IDE) to ensure no personal data collection.
- Complied with cookie-consent templates aligned with GDPR and CCPA.
- Delivered a compliant launch on schedule.
Campaign Summary & Performance
A mobile-based radial geo-targeting strategy raised awareness among healthcare professionals attending a major GI cancers conference.
- Early start impact: strong pre-event visibility as attendees arrived.
- Engagement spike on peak conference day, yielding the highest combined clicks across campaigns.
- CTRs remained within or above standard awareness benchmarks throughout the event.
- Top-performing creatives aligned closely with the event audience and drove the most efficient engagement.
Detailed performance dashboards captured clicks, impressions, CTR, and position trends for audit and optimization.


